Blog: CHEM 1305

1305 blog
Page Index
Exam 1
General Comments
- Some of the information covered on Exam 1 might best be considered of the general science sort, and some is purely chemistry in nature. Topics covered on Exam 1 include:
• measurements — esp. significant figures
• dimensional anaysis • classifications of matter
• elements, atoms, and ions
• the mathematical relationships among protons, neutrons, electrons, and charge
- View, learn, and understand all information in the instructional videos (each of the three topics are covered in detail) provided in the Exam 1 playlist.
Exam 2
General Comments
- The exam covers, for the most part, three topics:
• nomenclature
• balancing chemical equations
• types of reactions
- View, learn, and understand all information in the instructional videos (each of the three topics are covered in detail) provided in the Exam 2 playlist.
Nomenclature
- Biggest pitfall is using the wrong rule for a particular compound.
- Note the abundance of information in the
Nomenclature blog, especially
• nomenclature, 4-section organizational grid
• flow diagram for appling nomenclature rules
• acid "family" relationships
• Type I metals indicated in green should be menorized.
— be able to distinguish Metals from Non-metals
— remember, unless otherwise noted on the exam, any metal which is NOT a Type I metal may be assumed to be a Type II metal - Work the 40 questions in the Practice Set. (Answers provided)
Balancing Chemical Equations
- See guidance provided in the BCE (Balancing Chemical Equations) blog.
Types of Chemical Reactions
- Note that the 6 provided reaction types fall into two general, and very distinct categories:
• ionic reactions
• redox reactions - See guidance provided in the Reaction Types blog.
Exam Tripwires
- Multiple Reaction Type Classifications for Given Reaction: When classifying a given reaction, know that it will most likely fall into more than one class.
— For example, a double displacement reaction in which a precipitate forms falls into at least three categories: ① ionic, ② double displacement, and ③ precipitation. A multiple choice question might have one, two, or three of these as choices. - Memory Items Failure: test taker failed to memorize the following:
— 5 always-soluble ions (Na+, K+, NH4+, NO3–, CH3CO2– or C4H3O2– )
— 7 strong acids (HI, HBr, HCl, HClO3, HClO4, H2SO4, HNO3)
— 8 strong bases (LiOH, NaOH, KOH, RbOH, CsOH, Ba(OH)2, Sr(OH)2, Ca(OH)2 )
— 7 nomenclature ic-acids (acetic acid, carbonic acid, nitric acid, sulfuric acid, phosphoric acid, chloric acid, chromic acid)
— 2 exceptional ions (hydroxide, HO– or OH–; ammonium NH4+) - Mis-application of Concepts:
— Applying Ionic reaction concepts to REDOX reactions; and applying REDOX concepts to Ionic reactions.
— Applying the wrong nomenclature rule to a type of compound. For example, naming a Type II compound using the Type III rule.
Exam 3
General Comments
- The exam covers, for the most part, three topics:
• Stoichiometry
• Chemical composition
• Molarity
- View, learn, and understand all information in the instructional videos (each of the three topics are covered in detail) provided in the Exam 3 playlist.
Exam 4
placeholder
- item_1
- item_2
Final Exam
(Exam 5)
placeholder
- item_1
- item_2
Miscellaneous Student Questions
Maybe you have a curiosity of the types of questions other students ask, or maybe you would like to peruse the answers to some of those questions perchance you find a diamond in the rough. Regardless, and in no particular order (other than when received), are answers to some of those questions. Questions are selected on the basis of potential value to other students. Requestor identies are not provided, and some of the questions are paraphrased for efficiency or clarity.
TOPIC: Sig Figs
Hello Professor Stephenson,
I understand significant figures however I do have one quick question. When I am answering any type of number problem throughout the semester, do I need to always apply the significant figures rules? For example, if I am solving a temperature problem, do I need to make sure my answer lines up with the significant figures within the problem?
In the lab, yes. Definitely — a key component of "science" of properly recording data and calculations to the correctly level of significance.
In the lecture, most of what you will come across are multiple choice questions, and in that case — unless it's specifically a significant figure question — the answer choices are usually so far apart that whether the sig figs are reported to the first, second, or third decimal place is irrelevant. For example, if the answer choices are (a) 51 or (b) 22, it doesn't really matter whether you calculate '51.1' or '50.98... the best answer choice is clearly choice (a) and not (b).
But again, for lab work, or fill-in-the-blank, the sig figs need to be "spot-on" correct.
TOPIC: Stoichiometry; dimensional analysis
I have a question for you.
If I am going from Mol of 4NH3 to molecules NO2 do I skip O2? Pretend like O2 is not there, or do I add it to my dimensional analysis?
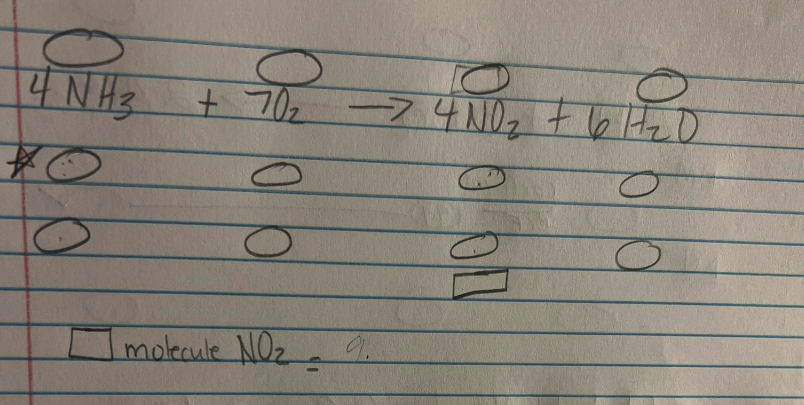
Skip O₂... you can skip the things not specifically in your question AFTER the chemical equation is balanced. Your DA [Dimensional Analysis] equation only needs ★ (ammonia), ▢ (nitrogen dioxide), and the conversions in between.
TOPIC: description
Q_grey
answer
TOPIC: description
Q_green
answer
