Blog: Solubility Table
The Solubility Table provides an easy way to determine if a material will become a solid and precipitate from water, or whether it will remain soluble and stay in the water.
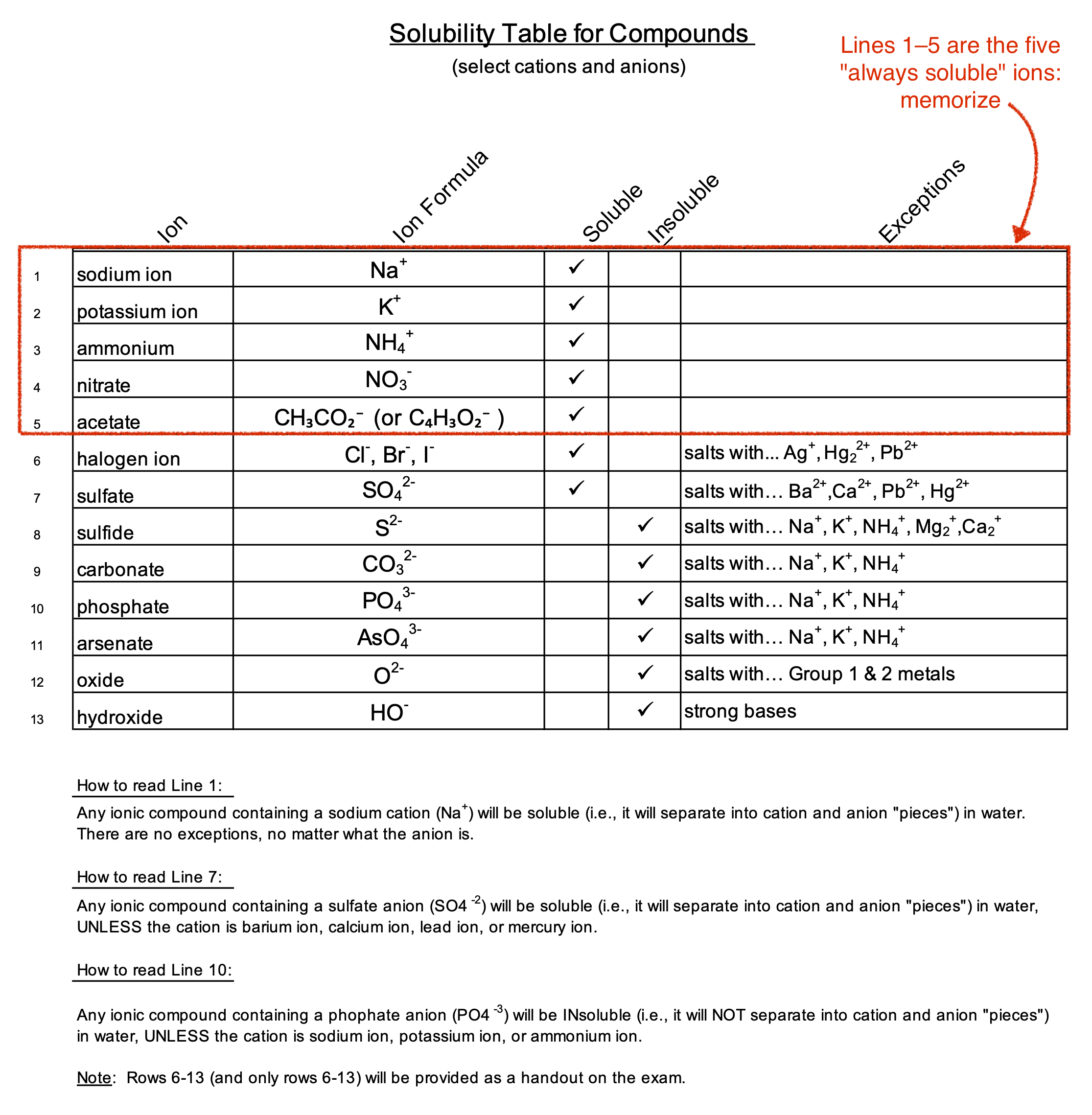
You have been provided a limited Solubility Table in your course Cheatsheet (see link along bottom edge of this page to find the cheatsheet for your class); however, for purposes of discussion, a 'full' table is provided below (within the green frame), along with instructions as to how to use it. Note that the top five lines — the so-called five "Always-Soluble Ions" — are not on the exam Cheatsheet version, nor are the written instructions.
The Five Always Soluble Ions
The five always soluble ions to memorize are:
- sodium ion: Na+
- potassium ion: K+
- ammonium ion: NH3+
- nitrate ion: NO3–
- acetate ion: CH3CO2– or C4H3O2– (two versions of same formula)
You may assume any ionic material made from any one of these ions will not precipitate from solution. For example, you may assume that sodium chloride, sodium fluoride, sodium phosphate, sodium carbonate, or sodium anything will not precipitate from aqueous solution.
Solubility Table Examples
A few examples illustrating the use of the solubility table:
- Sodium ion (Line 1)
- Looking across Line 1, the "Soluble" column is checked, which means that any ionic compound which includes a sodium ion is assumed to be soluble in water.
- But there may be exceptions, and so the "Exceptions" column must be checked before making a final decision.
- In this case, the "Exceptions" column is blank, and so there are no expections.
- Bottom line: Any ionic compound containing a sodium cation (Na+) will be soluble in water. That is to say, it will separate into sodium ion and anion "pieces", and those pieces will remain in the water phase.
- Sulfate ion (Line 7)
- Looking across Line 7, the "Soluble" column is checked, which means that any ionic compound which includes an SO4–2 ion is assumed to be soluble in water.
- But there may be exceptions, and so the "Exceptions" column must be checked before making a final decision. And indeed, on inspection it is found that there are four exceptions!
- The four exceptions are the positive charged ions of:
- barium (Ba2+),
- calcium (Ca2+),
- lead (Pb2+), and
- mercury (Hg22+).
- Bottom line: Any ionic compound containing a sulfate anion (SO4–2) will be soluble, UNLESS the sulfate combines with a barium cation, calcium cation, lead cation, or mercury cation, in which case the new material will precipitate.
- Phosphate (Line 10)
- Looking across Line 10, the "Insoluble" column is checked, which means that any ionic compound which includes an phosphate ion (PO4–3) is assumed to be INsoluble in water. That is, it is not soluble and will therefore precipitate.
- But there may be exceptions, and so the "Exceptions" column must be checked before making a final decision. Indeed, there are three exceptions!
- The exceptions are ions of:
- sodium (Na+),
- potassium (K+), and
- ammonium (NH3+).
- Bottom line: any ionic compound containing, or made from, a phophate anion (PO43–) will be INsoluble in water (will form a precipitate), UNLESS the cation is sodium ion, potassium ion, or ammonium ion.
⚠️ Disclaimer: solubility is a complex phenomenon, and the application in this course limited to very clear-cut and simplified cases involving relatively few ionic compounds. The idea being for you to learn the basic concepts of the phenomenon, rather than become a master at solubility determination and calculations.
