Blog: Nomenclature
Page Index:- Nomenclature Video Library
- Grid
- Pathway
- Acids to Memorize ("memory acids")
- Acid Families
- Type I Metals (memorize)
- Practice Set
Nomenclature Video Playlist
A list of videos which will explain just about everything you need to know about nomenclature for this course → NOMENCLATURE VIDEOS
See Videos 5.01 – 5.15.
Key diagrams from those videos are reproduced below, as is a practice page.
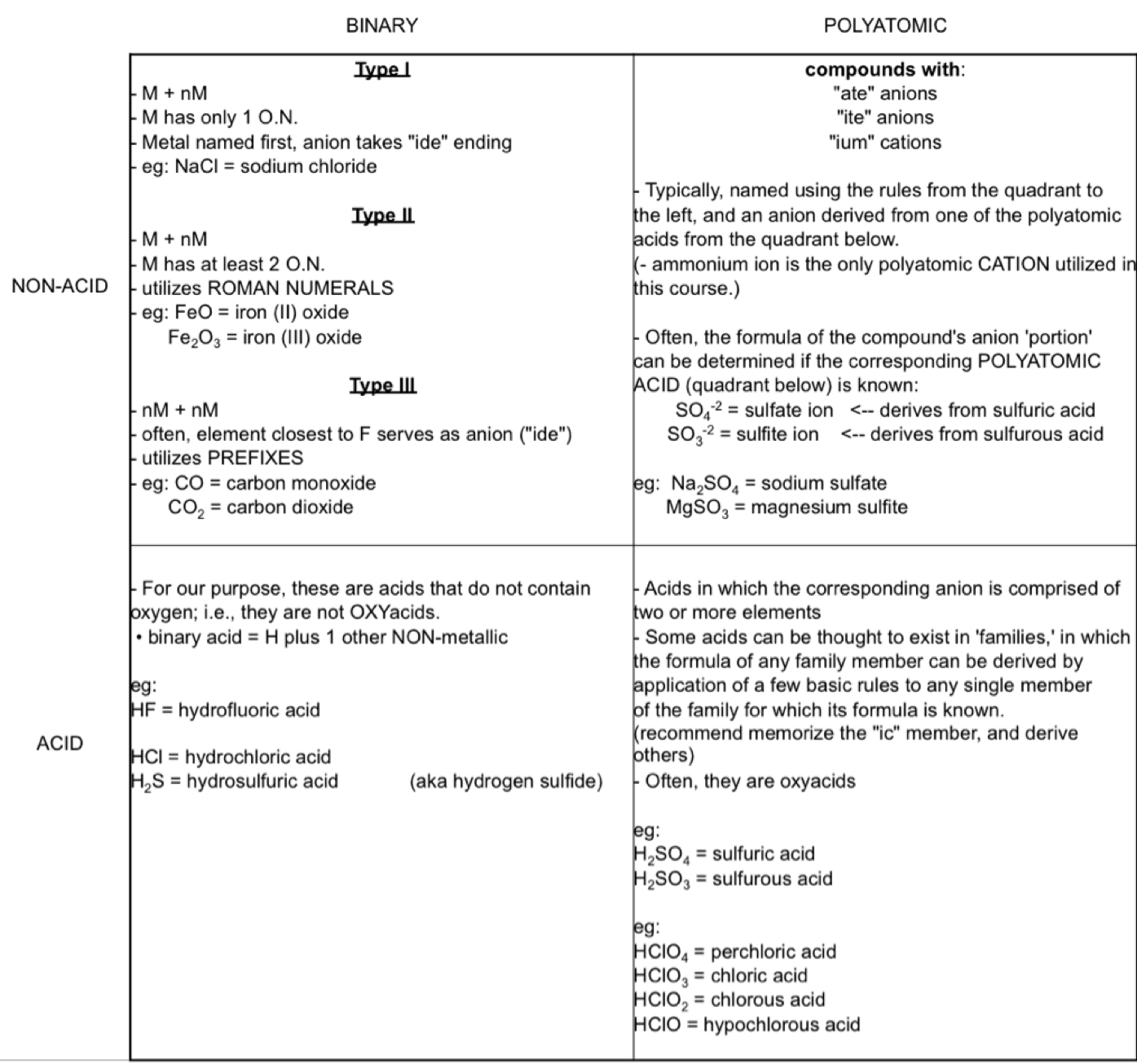
Nomenclature Grid
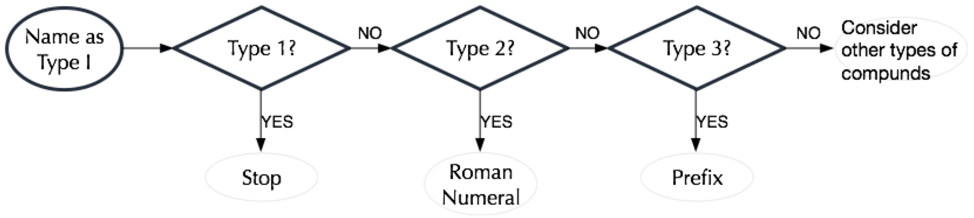
Nomenclature Pathway
Memory Acids
- nitric acid — HNO3
- sulfuric acid — H2SO4
- chromic acid — H2Cr2O4
- phosphoric acid — H3PO4
- chloric acid — HClO3
- carbonic acid — H2CO3
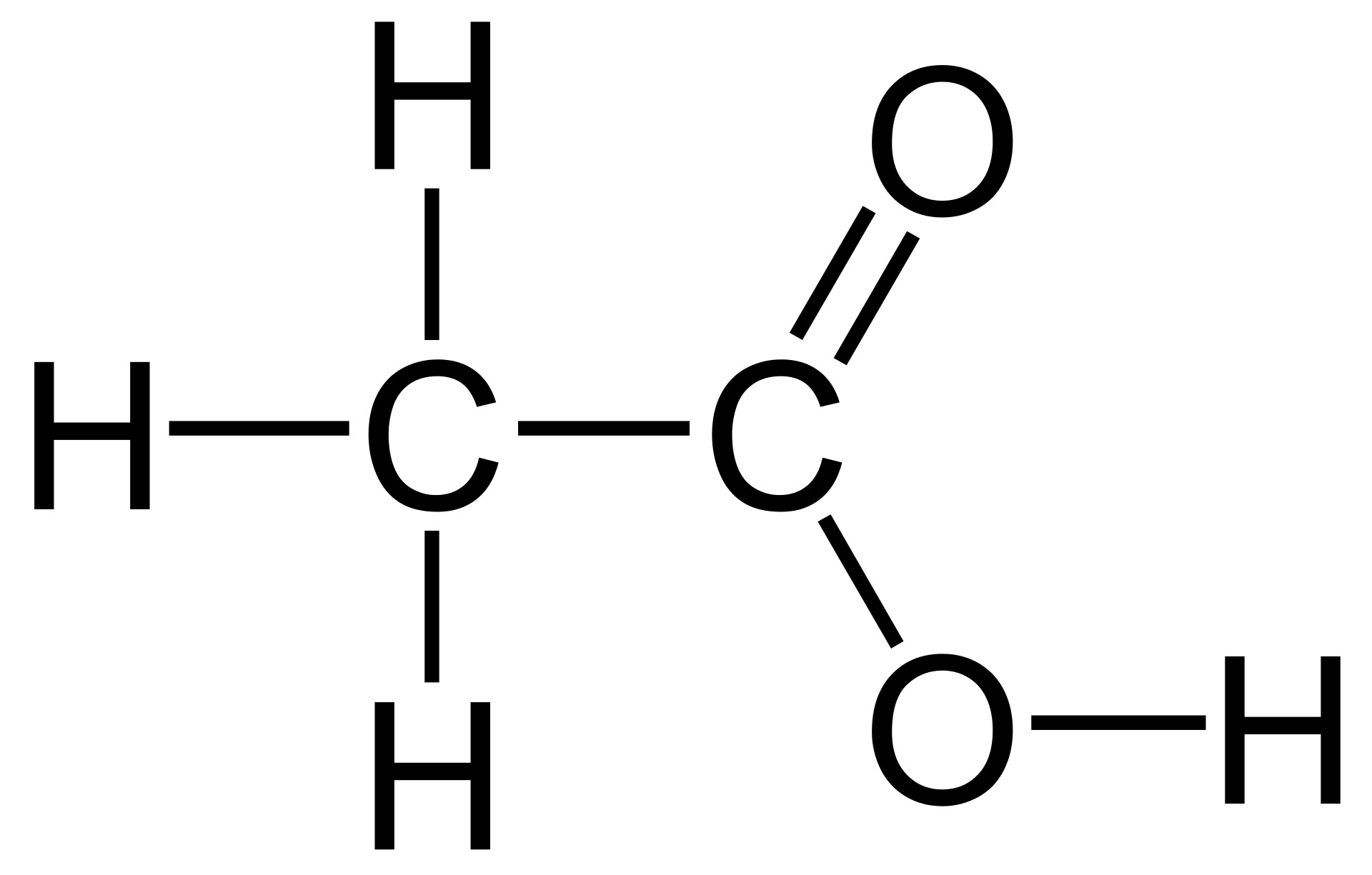
- acetic acid — CH3COOH –or– CH3CO2H –or– C2H4O2
... regarding acetic acid, shown are three versions of the same compound... note that each formula contains two carbons, four hydrogens, and two oxygens... in 2-D, the formula for acetic acid is shown immediately below. The three "1-Dimensional" formulas are attempting to convey different information about the same 2-Dimensional structure.
Acid Families
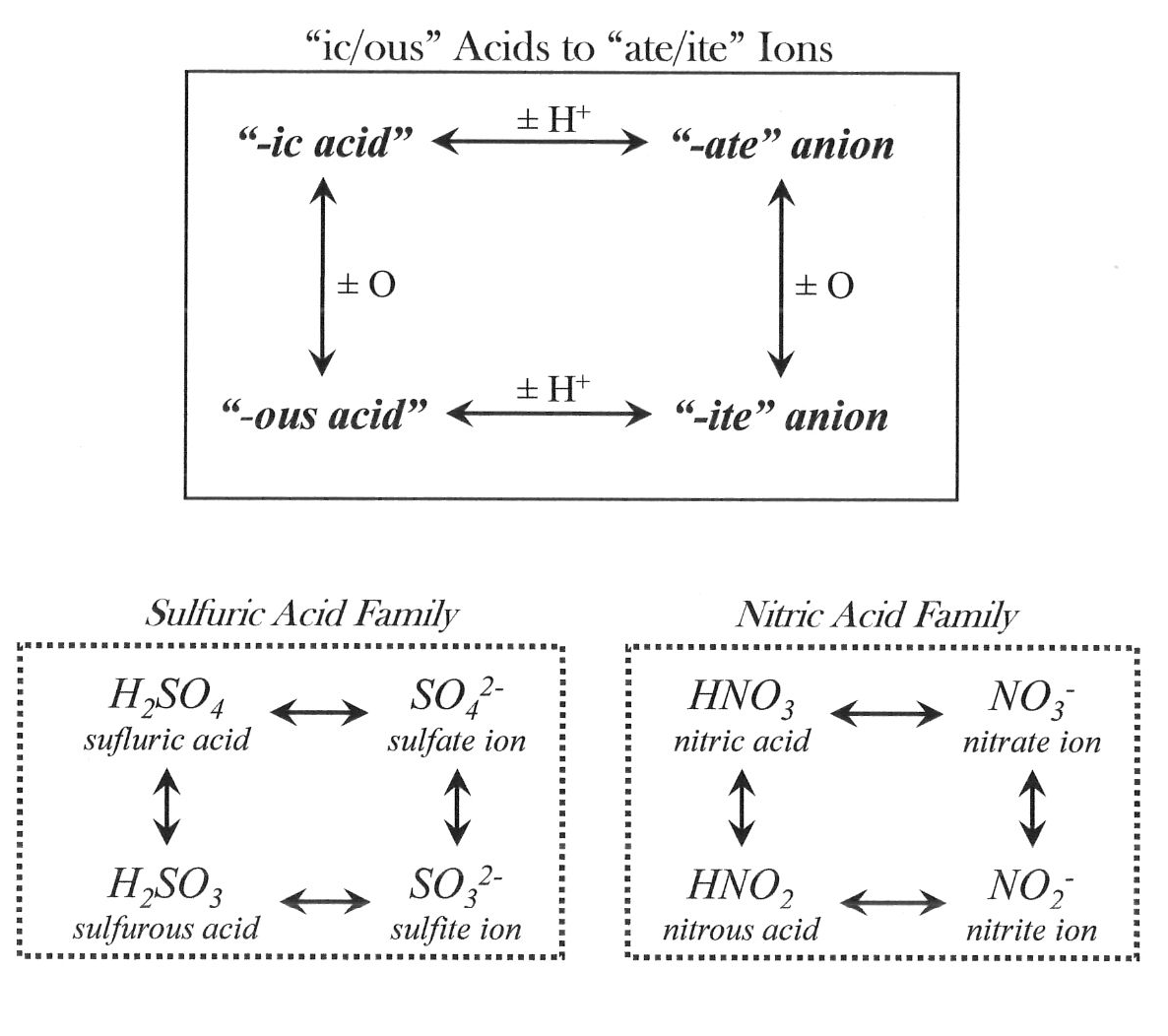
- extended family — denotes acid with ic acid, ate, ous acid, and ite variants, as demonstrated in the boxes ... includes nitric, sulfuric, chromic, and phosphoric acids
- super family — denotes extended family acid which also has per_ic acid, per_ate, hypo_ous acid, and hypo_ite variants ... includes chloric, bromic, and iodic acids
- orphaned — denotes acid with ic acid and ate variants only... includes carbonic and acetic acids
Remember that anions derived from polyatomic oxyacids — those things ending in ate, per_ate,ite, hypo_ite — are named as a single group, and not as individual atoms.
For example, PO4– is not tetraoxide phosphide ion (treating P and O as separate things), rather it is a single phosphate ion (treating PO4 as a single unit).
But to identify polyatomic ions derived from acids as a single unit, you must necessarily know the polyatomic acid from whence the anion came.
Type I Metals
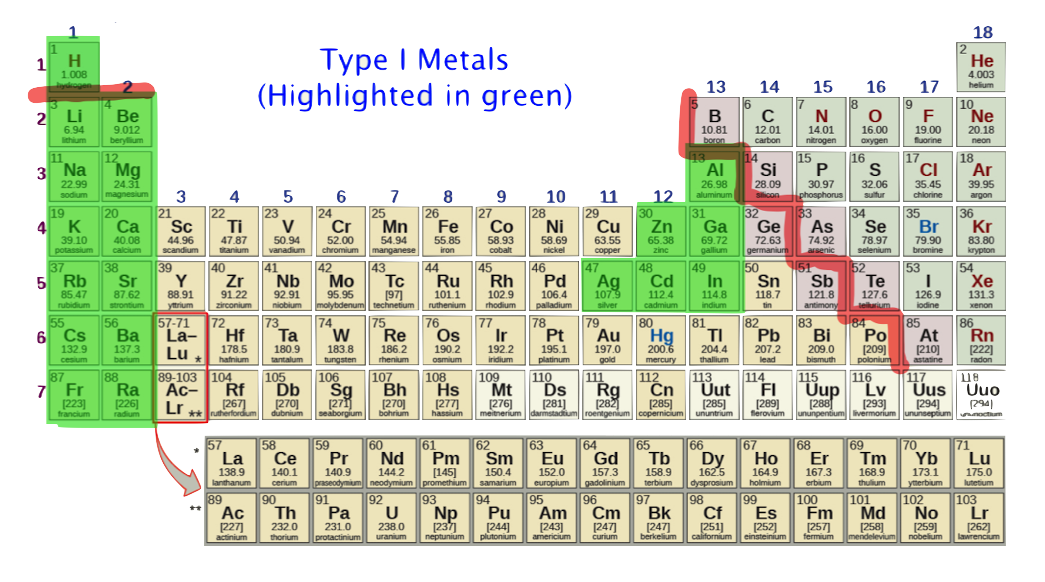
Type I Metals
- Type I metal — denotes a metal with only one oxidation number. That is, upon ionization, the metal will always produce an ion of the same charge. For example, upon ionizing, Ca will only produce Ca2+; and never Ca+ , Ca3+ , Ca4+, etc. In short, for calcium it's Ca2+ or nothing.
Type I metals are highlighted in green. These should be memorized. Type I metals which are not highlighted in green need not be memorized: information will be provided as needed.
Type II Metals
- Type II metal — denotes a metal with two or more oxidation numbers. That is, upon ionization, the metal has the option to produce ions of different charges. For example, Fe can ionize to Fe2+ or Fe3+. Since iron has at least two choices of ions into which it may change, it falls into the class of Type II metal. Any metal with two or more choices of ions possible is referred to as a Type II metal.
When naming an ion derived from a Type II metal, the charge of the ion is designate with Roman Numeral. In contrast, the charge on a Type I metal does not carry a roman numeral designation. In the following examples, note that the use of roman numerals is strictly limited to Type II metals:
- Ca2+ — calcium ion
- Fe2+ — iron(II) ion
- Fe3+ — iron(III)ion
- Na+ — sodium ion
- Cu+ — copper (I) ion
- Cu2+ — copper (II) ion
- Ag+ — silver ion
- Au+ — gold(I) ion
- Au3+ — gold(III) ion
🚩 Bottom line: if you place a roman numeral in the name of an ionic compound derived from a Type I metal, the name is probably wrong; if you fail to place a roman numeral in the name of an ionic compound derived from a Type II metal, the name is likewise probably wrong.
Practice Set
(answers below)


