Blog: Lewis Dot Structures & Molecular Structure
Page Index:- Blog Introduction
- Electronic and Molecular Geometries Interdependence (for linear, trigonal planar, and tetrahedral)
- Q&A
Blog Introduction
This blog addresses Lewis Dot Structures & Molecular Structures for both IntroChem (CHEM1305) and General Chemistry (CHEM1411). Unless otherwise noted, assume the information given is relevant to both courses. Classes specifically targeting General Chemistry will be designated with a "GenChem" or "CHEM1411" label.
IntroChem vs GenChem
The basic difference between the two courses is that IntroChem is limited to central atoms with no more than four bonding/long pairs, whereas GenChem also includes central atoms with five or six bonding/long pairs. Put another way, IntroChem includes electronic geometries up to tetrahedral shape; whereas GenChem includes electronic geometries up to octahedral shape.
Electronic & Molecular Geometry Interdependence (IntroChem/CHEM1305)
The following chart shows the flow from a molecule's electronic geometry (EG) to its possible molecular geometries (MG). This chart is limited to molecules with a molecular geometry of tetrahedral or smaller — as is the case for Introductory Chemistry.
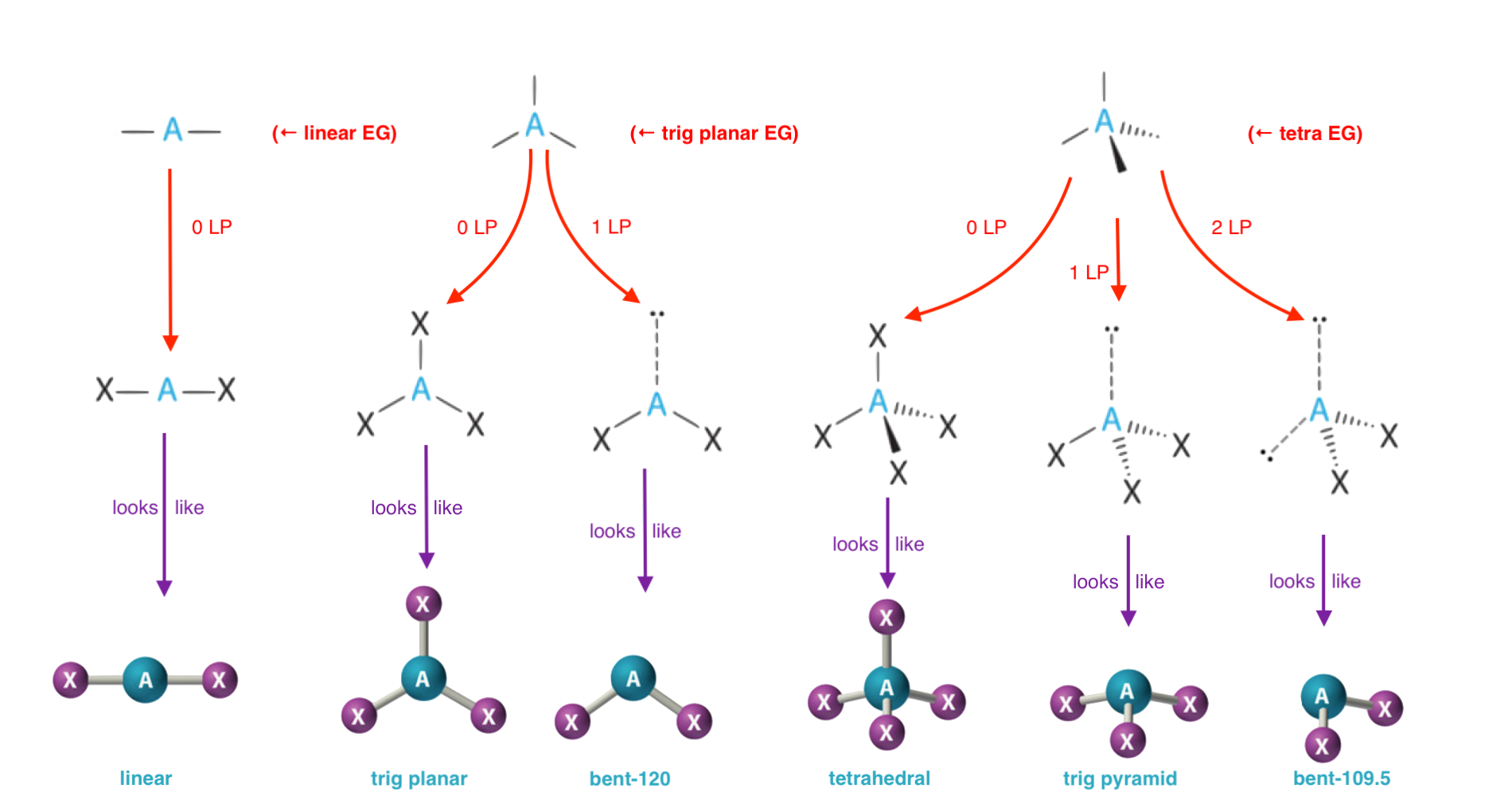
Q&A
Q: CHEM-1411: Question about Lab K
Dr. Stephenson,
For the report form for [CHEM 1105] lab K, in the polarity box, what does it mean when it asks for the same end group?
A: A molecule is 'polar' when either of the following occur:
- at least one of the end groups on the central atom is different from any of the others —OR—
- the molecular geometry is different from the electronic geometry
(Corollary: it it passes both, then it's non-polar)
